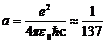Natural constants: the fixed values of certain physical quantities in the ►laws of nature.
Natural constants are regarded as eternal and fundamental. They are supposed to have the same value in any place and at any time in the universe and, within the framework of the theory that postulates them, cannot be derived from other laws of nature than the ones in which they explicitly occur. This is why a scientific theory is regarded as stronger the fewer natural constants it requires. Thus, for example, the radius of the hydrogen atom was still regarded as a natural constant in the early 20th century but later could be derived from Planck's constant (see below) together with the ►speed of light, electron charge, electron mass, and proton mass.
There are hypotheses according to which certain natural constants have changed in the course of the development of the universe, which is as much as to say that they weren't constants in the first place. Some ►many world theories postulate different values for natural constants in the different variants or regions of the universe. The natural constants currently known to hold in our universe possess the exact values that are required for biological life to exist. This fact is reflected in the ►anthropic principle. The most important natural constants are:
|
3 |
Number of ►dimensions of space. |
|
48 |
Number of elementary particles according to the standard model. |
|
299,792,458 m/s |
Speed of light in a vacuum. |
|
4π ∙ 10-7 N/A2 |
Permeability of vacuum: magnetic flux density as a function of the strength of the magnetic field. |
|
8.854187817 ∙ 10-12 F/m |
Relative static permittivity of vacuum: electric flux density as a function of the strength of the electric field. |
|
6.67242 ∙ 10-11 m3/s2kg |
Constant of gravitation: attraction of masses as a function of their distances. |
|
1.3806505 ∙ 10-23 J/K |
Boltzmann constant: energy per temperature change in ideal gases (see ►Entropy). |
|
1.60217653 ∙ 10-19 C |
Electric charge of electrons. |
|
9.1093826 ∙ 10-31 kg |
Rest mass of electrons. |
|
1.67262171 ∙ 10-27 kg |
Rest mass of protons. |
|
6.0221415 ∙ 1023 /mol |
Avogadro constant: number of atoms in 12 grams of carbon. |
|
6.6260693 ∙ 10-34 Js |
Planck's constant: energy of a light particle as a function of its oscillating frequency. Basis of the Planck units. |
|
1/137.03599976 |
Fine-structure constant: coupling strength between light particle and electric charge.* |
*This is not, strictly speaking, a fundamental constant, since it can be derived from the speed of light c, electric flux density ε0, electric charge e and Planck's constant ħ.